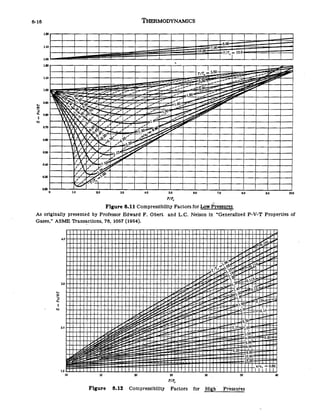
- Home
- compressibility factor equation
- 117. Compressibility factor H, behaving as rea gas is 1) 1 RTV 3) 1+- RT 4) (1-a) 18. If V is the observed molor unlum
117. Compressibility factor H, behaving as rea gas is 1) 1 RTV 3) 1+- RT 4) (1-a) 18. If V is the observed molor unlum
4.8 (595) · $ 24.99 · In stock
Click here:point_up_2:to get an answer to your question :writing_hand:117 compressibility factor for h behaving as reagas is1 1rtv31rt41a18 if v is the observed
Click here👆to get an answer to your question ✍️ 117- Compressibility factor H- behaving as rea gas is 1- 1 RTV 3- 1- RT 4- -1-a- 18- If V is the observed molor unlum
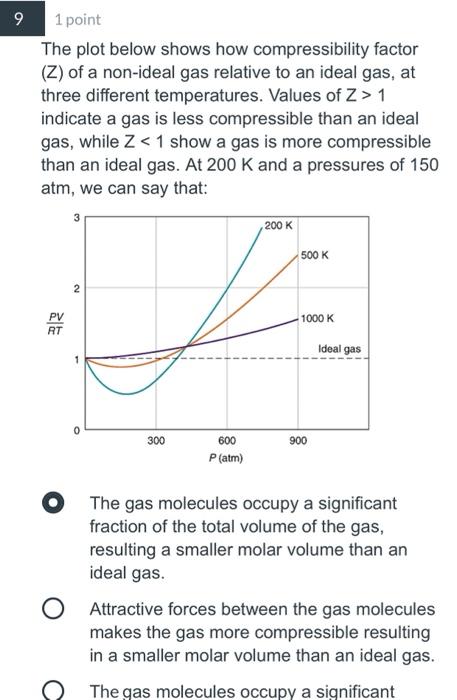
Solved 1 1 point If the root mean square speed of a gas

The compressibility factor a real gas high pressure is:1+ dfrac{RT}{pb}1+ dfrac{pb}{RT}11- dfrac{pb}{RT}

Carbon under pressure - ScienceDirect

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics
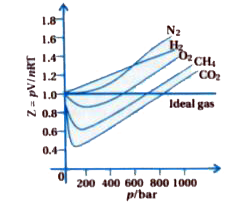
Gujrati] Explain compressibility factor (Z).

D) P V- (C) PV- nRT Compressibility factor H2 behaving as real gas is : D) Pb RTV (A) 1 RTV (1-a)

The compressibility factor a real gas is BP expressed by, Z=1+ er. The value of B 500 K and 600 bar is 0.0169 L/mol. Therefore the molar volume of the gas 500
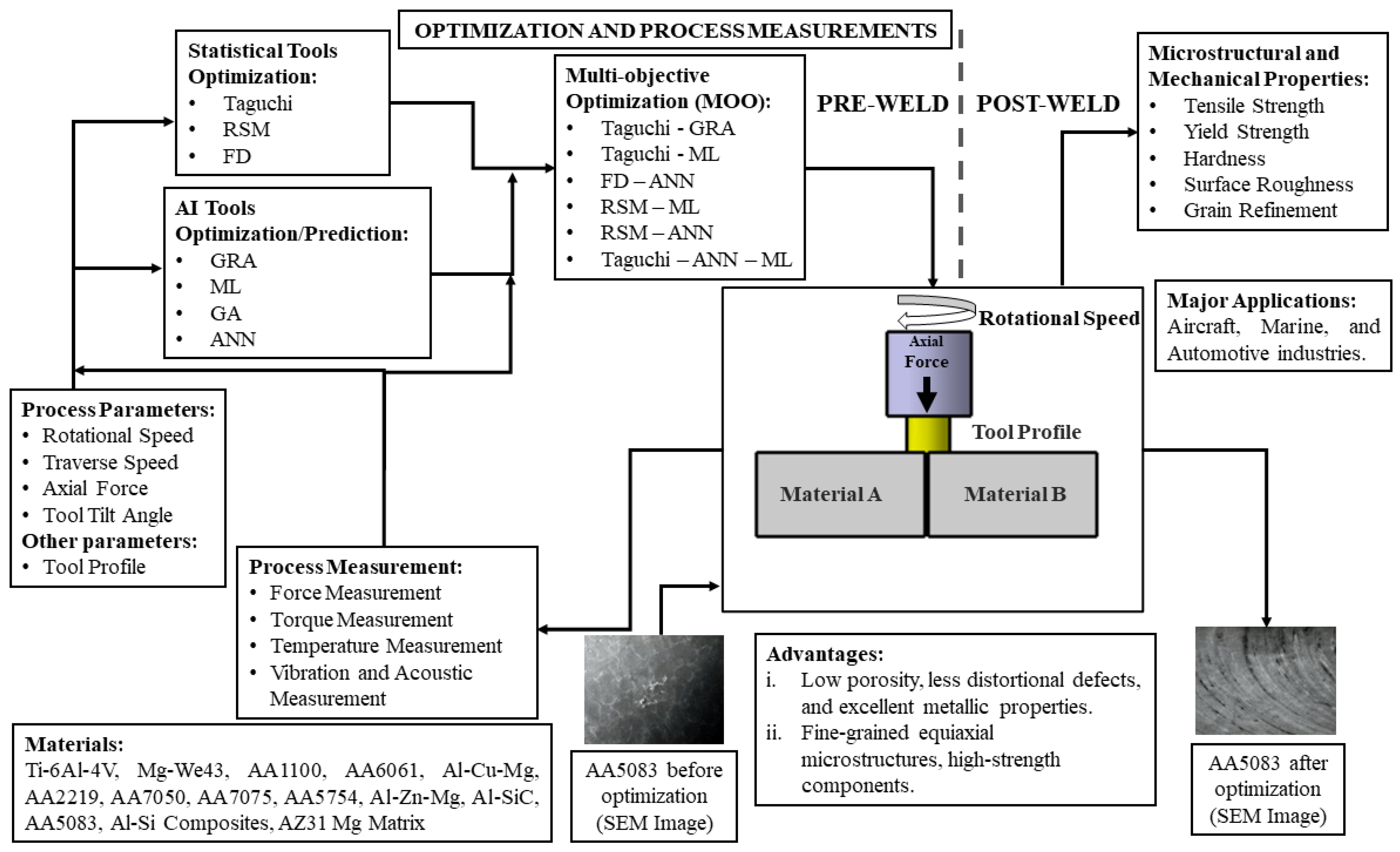
JMMP, Free Full-Text

quot; Who controls the vocabulary , controls the knowledge " - FBC>s

The compression factor (compressibility factor) for 1 mol of a van der