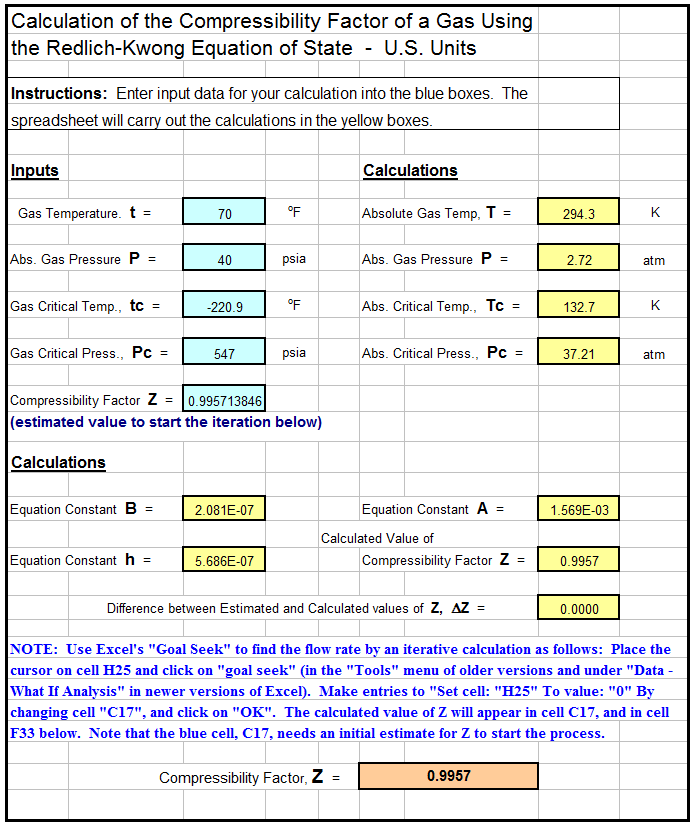
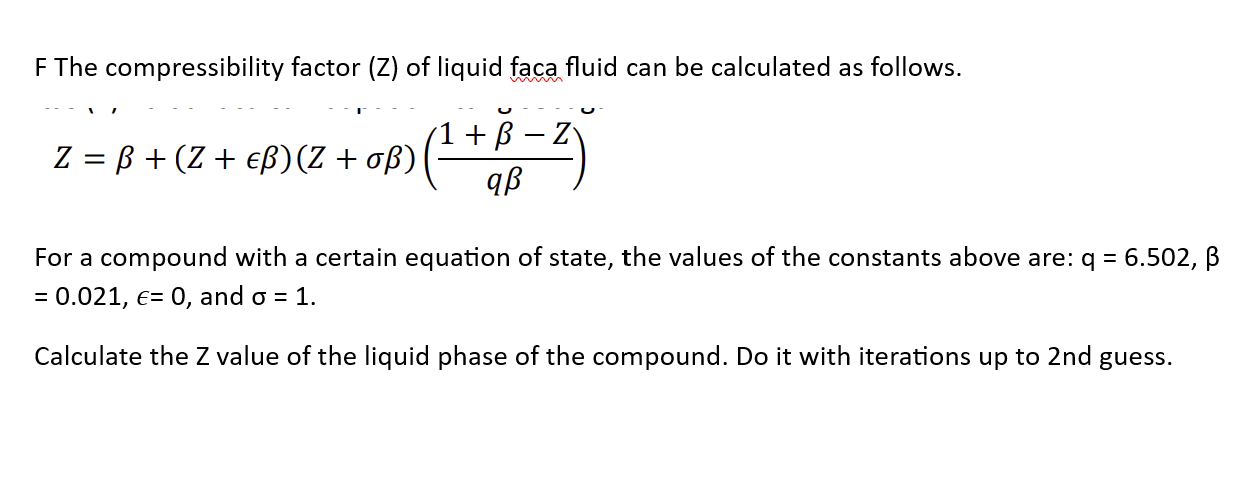
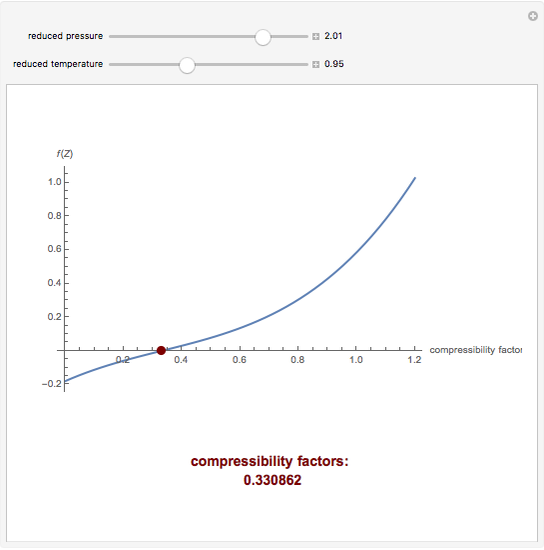
Developing a Thermodynamical Method for Prediction of Activity
4.7 (325) · $ 12.99 · In stock
Results of the experimental measurements on the partial molar volume of kerosene used as a medium for dissolving TBP are utilized to determine the activity of TBP in the binary kerosene-TBP solution through the application of Gibbs-Duhem equation. The treatment is based on combination of the experimental data with the thermodynamic values available on the compressibility factor of pure kerosene at room temperature. It is shown that the activity of TBP in kerosene has a positive deviation from ideality with an activity coefficient derived as follows:1) at X TBP ≤ 0.01: γ TBP = 42.530, 2) at the 0.01 X TBP 0.2: 3) at the higher TBP concentrations 0.2 X TBP 0.97: and 4) at TBP Raoultian concentrations 0.97 ≤ X TBP:γ TBP = 1. These quantities can be utilized at temperature closed to 298 K.

The integral molar volume of TBP-kerosene binary solution as a function

Extraction of ZN, MN and CO from ZN-MN-CO-CD-NI containing solution using D2EHPA, Cyanex® 272 and Cyanex® 302

Thermodynamic system which presents the balance of sources and types of
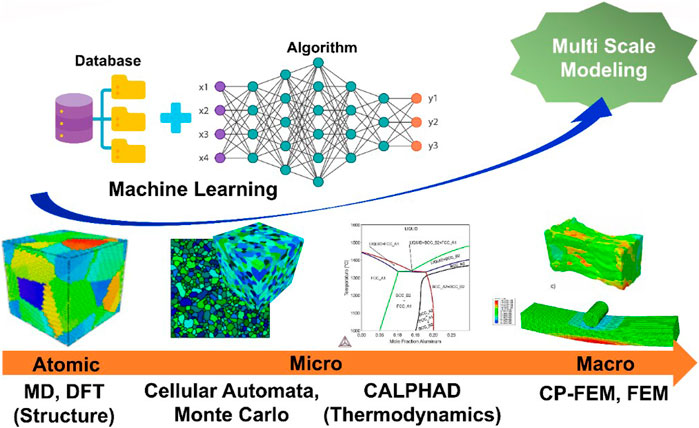
Frontiers Novel Alloy Design Concepts Enabling Enhanced Mechanical Properties of High Entropy Alloys

刊内检索-【维普期刊官网】- 中文期刊服务平台

The integral molar volume of TBP-kerosene binary solution as a function

Synergistic effect of MEHPA on co-extraction of zinc and cadmium with DEHPA

PDF) The performance of UNIFAC and related group contribution models Part I. Prediction of infinite dilution activity coefficients

5 Conclusions and Recommendations, Critical Issues in Weather Modification Research

PDF) Thermodynamics of extraction of Zn2+ from sulfuric acid media with a mixture of DEHPA and MEHPA

Predicting climate anomalies: A real challenge - ScienceDirect

Centre for Biotech Data Science :: Ghent University Global Campus