At 273 K measurements on argon gave B = -21.7 cm$^3$ mol$^{
5 (654) · $ 7.00 · In stock

The apparatus shown consists of three bulbs connected by stopcock
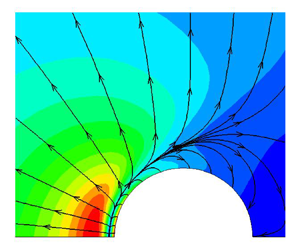
Kinetic modelling of rarefied gas flows with radiation, Journal of Fluid Mechanics
How does one find out the volume of a gas at STP? - Quora

Bipolar membrane electrolyzers enable high single-pass CO2 electroreduction to multicarbon products

Left) Argon adsorption ( fi lled symbols) and desorption (open

Pyrolysis of sulfonic acid substituted benzenes and investigation of CO2 capture capability of resulting carbons - ScienceDirect

Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law

One mole of argon is expanded according to process equation PV^(1.5)=c

SOLVED: At 273 K, measurements on argon gave B = -21.7 cm^3/mol and C = 1200 cm^6/mol^2, where B and C are the second and third virial coefficients in the expression of
-1.png)
Solved] The following financial statements apply
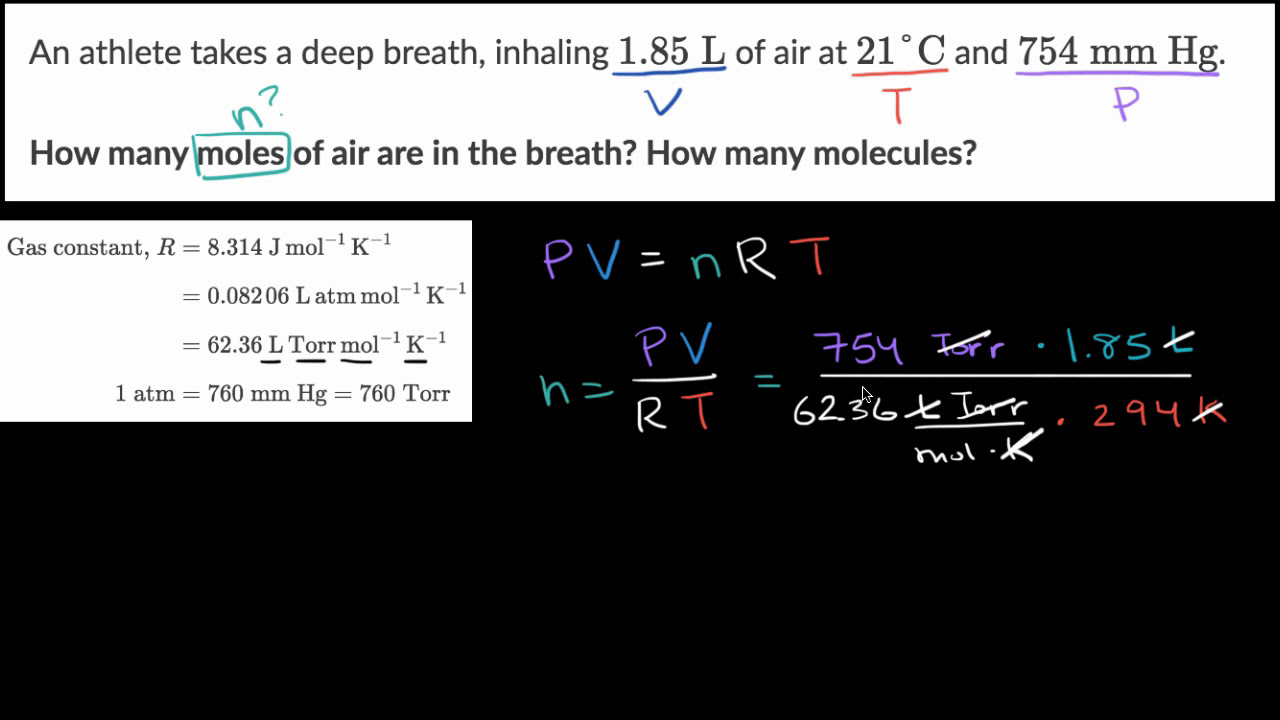
Using the ideal gas law to calculate number of moles (worked example) (video)

Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law












