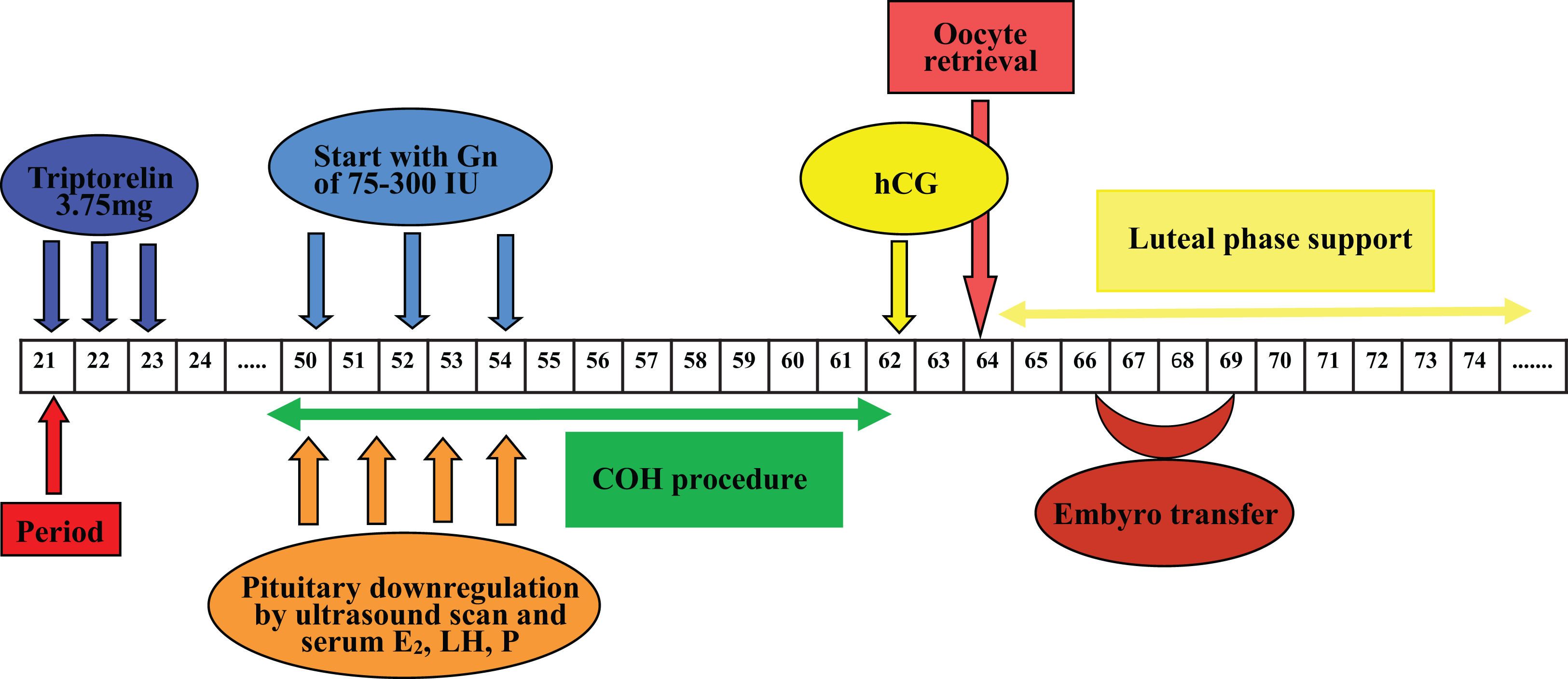
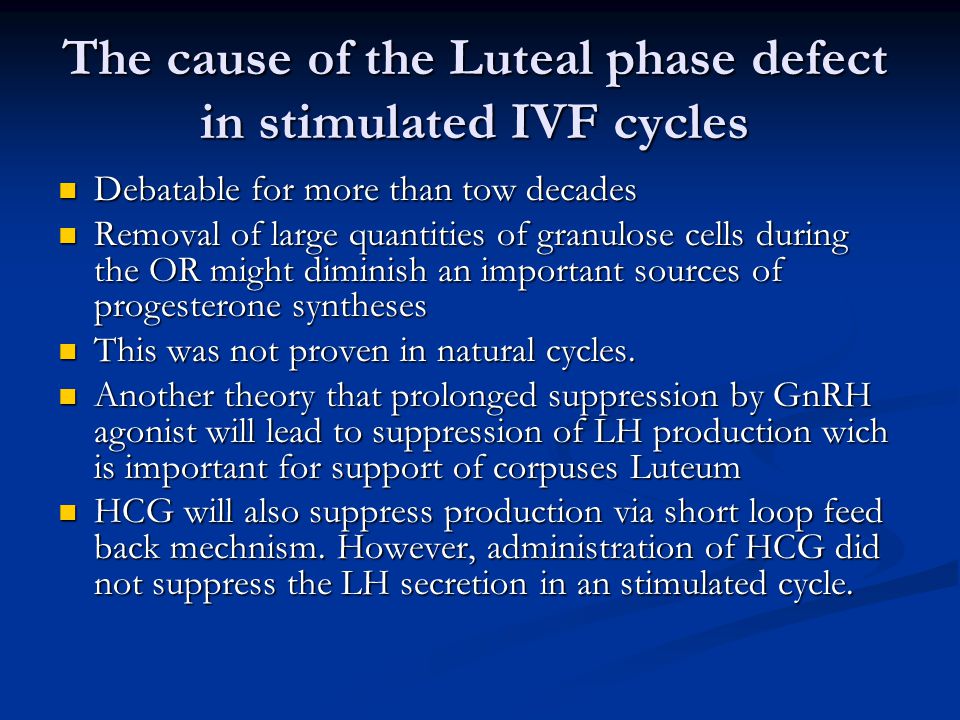
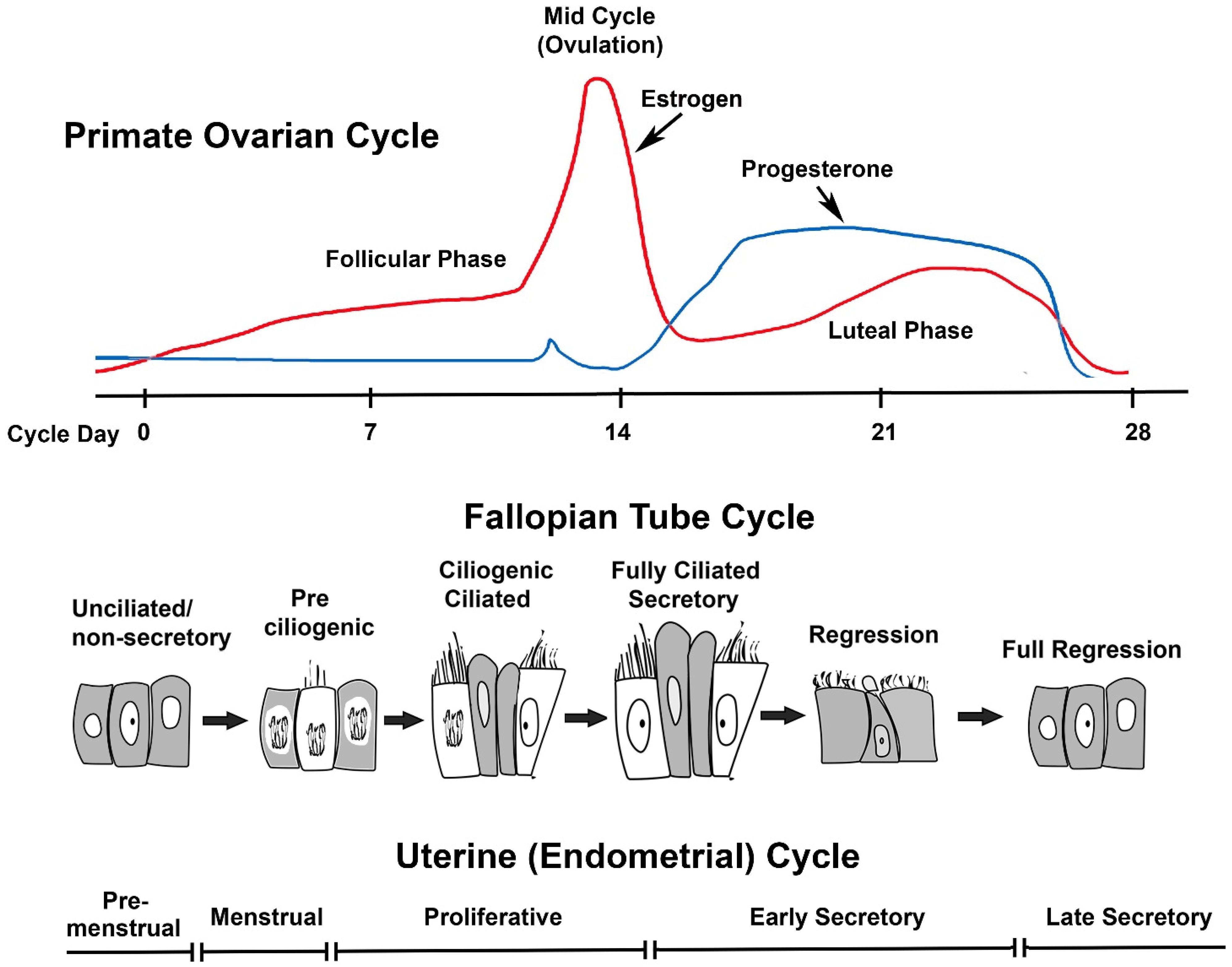
Therapies that target progesterone action hold potential as contraceptives and in managing gynecological disorders. Recent literature reviews describe the role of steroid hormones in regulating the mammalian oviduct and document that estrogen is required to stimulate epithelial differentiation into a fully functional ciliated and secretory state. However, these reviews do not specifically address progesterone action in nonhuman primates (NHPs). Primates differ from most other mammals in that estrogen levels are >50 pg/mL during the entire menstrual cycle, except for a brief decline immediately preceding menstruation. Progesterone secreted in the luteal phase suppresses oviductal ciliation and secretion; at the end of the menstrual cycle, the drop in progesterone triggers renewed estrogen-driven tubal cell proliferation ciliation secretory activity. Thus, progesterone, not estrogen, drives fallopian tube cycles. Specific receptors mediate these actions of progesterone, and synthetic progesterone receptor modulators (PRMs) disrupt the normal cyclic regulation of the tube, significantly altering steroid receptor expression, cilia abundance, cilia beat frequency, and the tubal secretory milieu. Addressing the role of progesterone in the NHP oviduct is a critical step in advancing PRMs as pharmaceutical therapies.

excel - How to view full contents if text is too large for the

BioTuring for Single Cell Discoveries
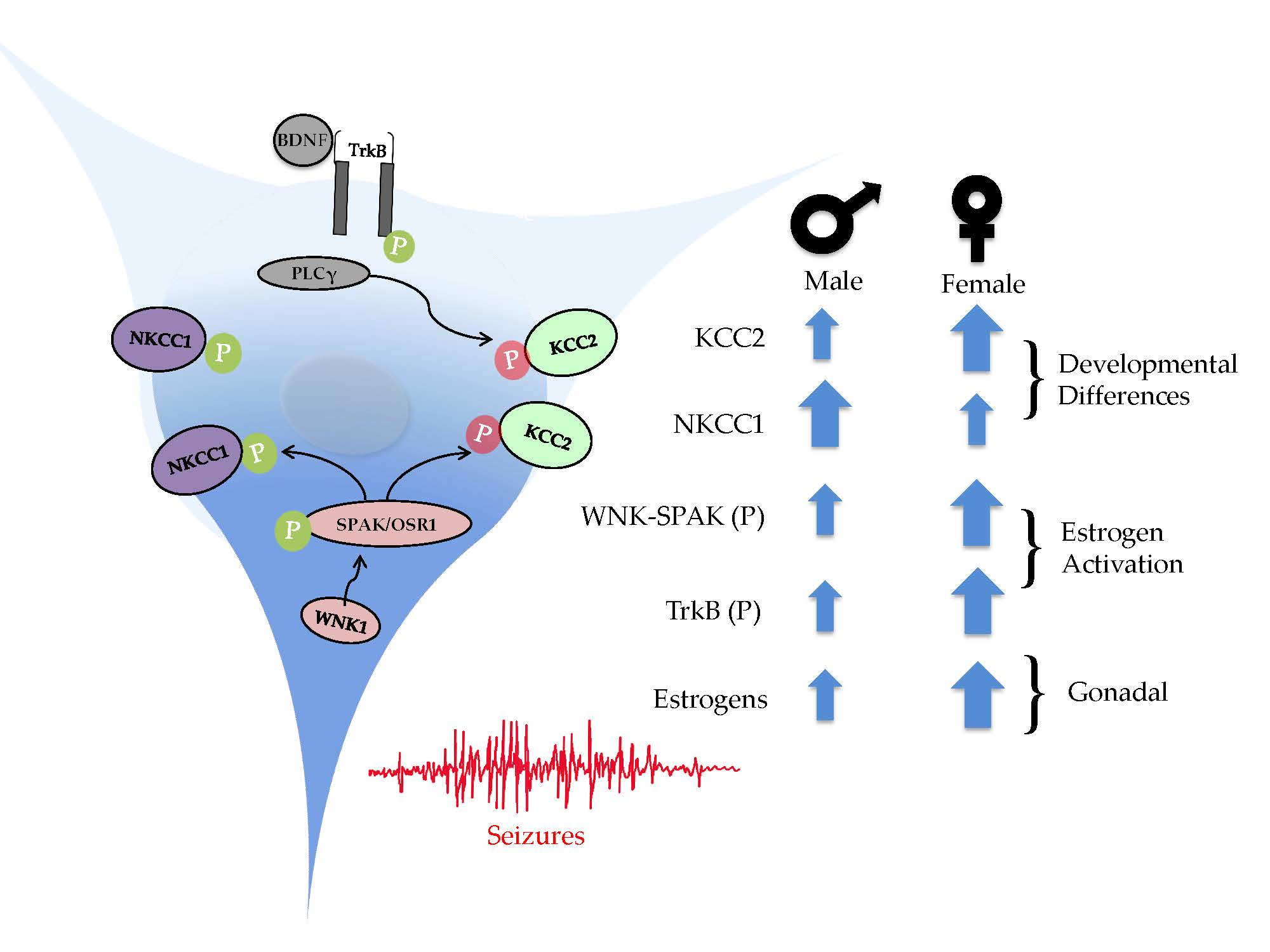
Cells Free Full Text Sex Dependent Signaling Pathways Underlying Seizure Susceptibility And 5187
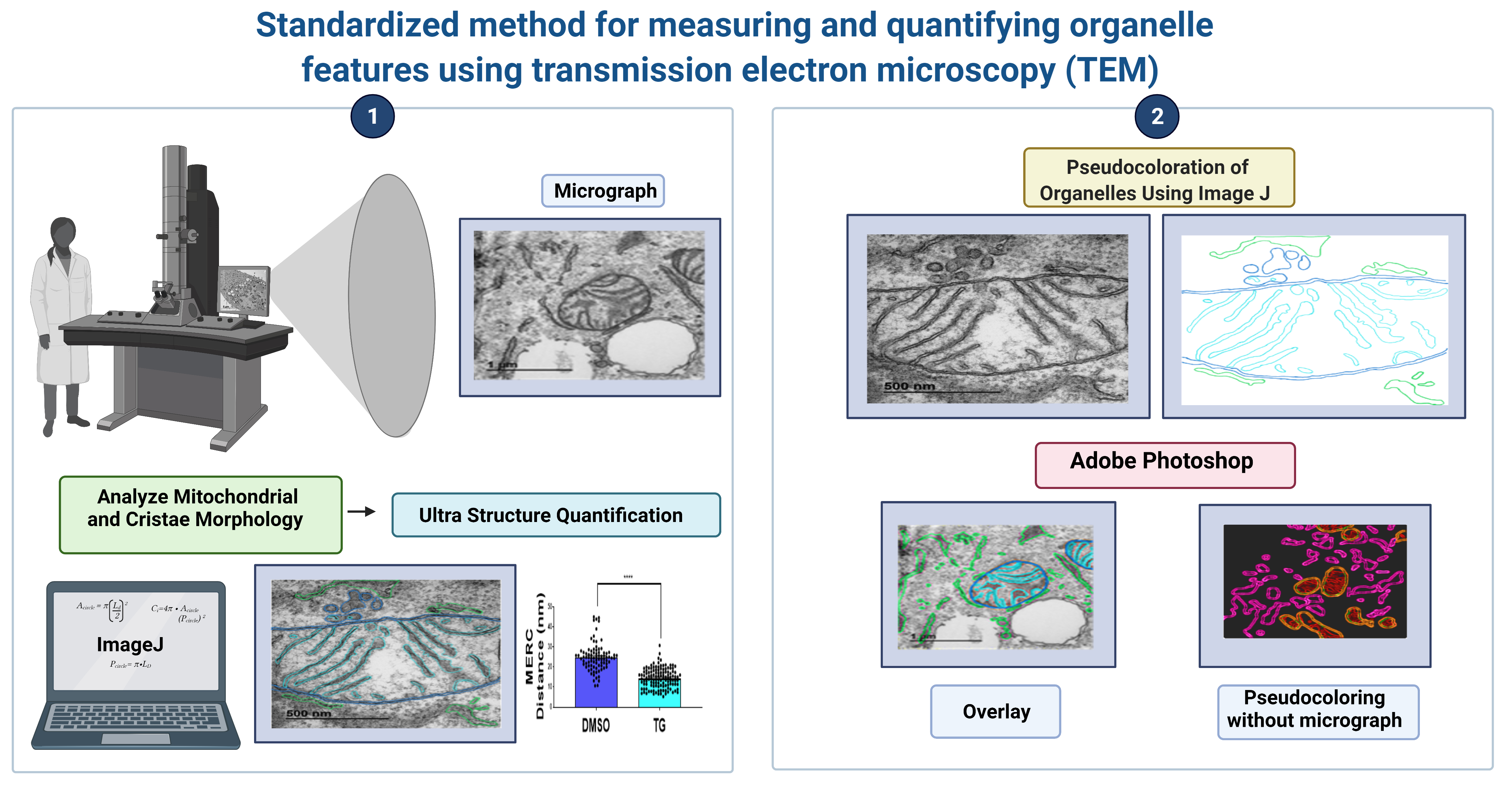
Cells, Free Full-Text

Cells, Free Full-Text

Nicole L Yuwono (@NicoleYuwono) / X

Cells, Free Full-Text, click desenvolvimento aec entrar

Label-free, full-field visualization of red blood cell (RBC
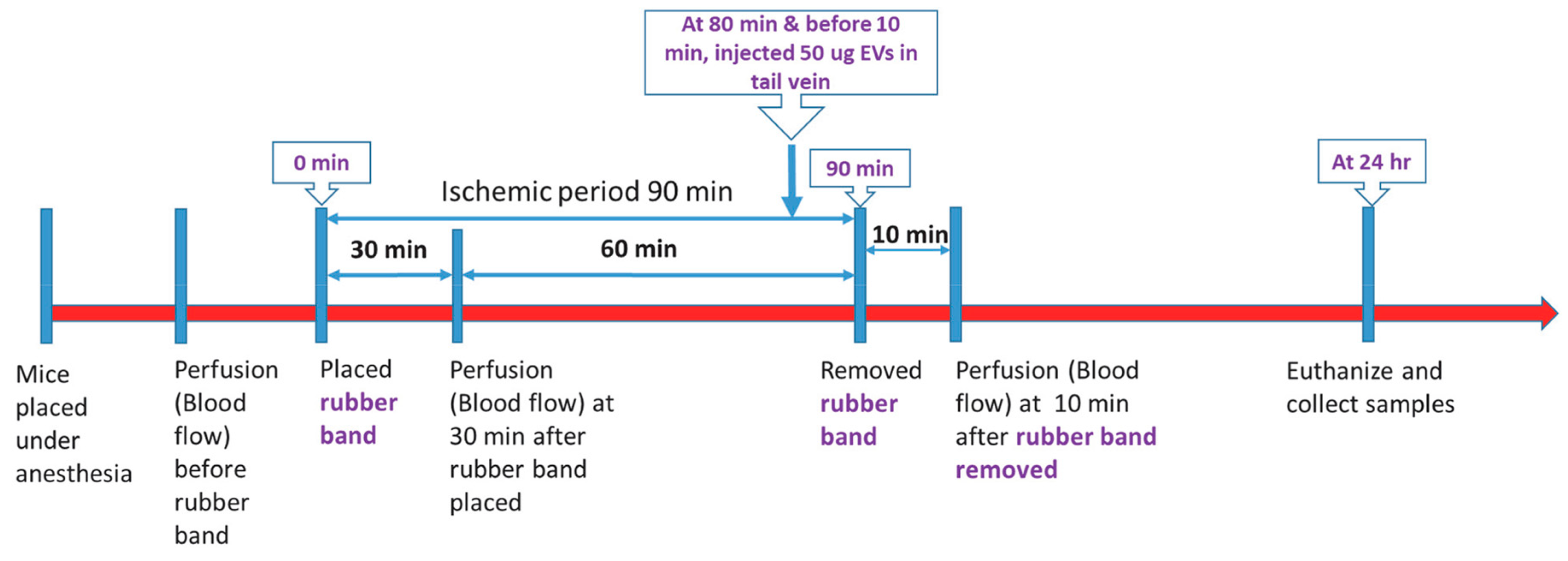
Cells, Free Full-Text


/etam-shapewear-unterteil-6535948-schwarz-0000302616244.jpg)