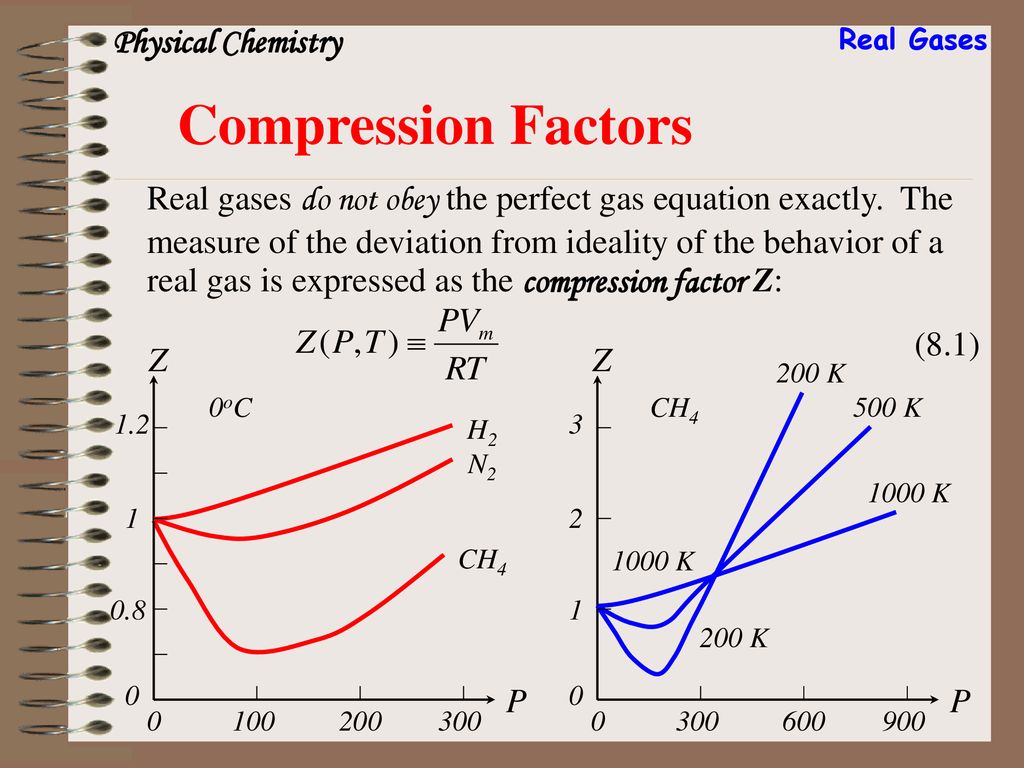
UNUB At Boyle temperature, the value of compressi factor Z has a
4.6 (482) · $ 13.99 · In stock
Click here:point_up_2:to get an answer to your question :writing_hand:unubat boyle temperature the value of compressifactor z has a value of one over a
Click here👆to get an answer to your question ✍️ UNUB At Boyle temperature- the value of compressi factor Z has a value of one over a wide range of pressure- This is due to the fact that in the van der Waals equation -1- The constant a is negligible and not b -2- The constant b is negligible and not a -3- Both the constant a and b are negligible -4- Attraction balances repulsion

The value of compressibility factor at the critical state the gas matches with the `Z_(c )` is

Solved The plot below shows how compressibility factor (Z)

and two-phase flow in singular geometries and safety relief valves

Deviation From Ideal Gas Behavior - Study Material for IIT JEE

Degrees Conferred by Major ( ) Source: National Center for Education Statistics (NCES) Business 366,815 Social Sciences 178,543 Psychology 108, ppt download

One mole of a real gas within the Boyle's temperature range shows a pressure of 3 atm in a container of V liter. The gas is heated to some higher temperature in

qph.cf2.quoracdn.net/main-thumb-30453142-200-bmcwo

Deviation From Ideal Gas Behavior - Study Material for IIT JEE

Physical Chemistry The Compression Factor (Z) [w/1 example]

Solved (b) The compressibility factor Z of a non-ideal gas

Txt.04 - Std'11 - Chemistry - Part-I by Saurabh Suman - Issuu
Why compressibility factor of areal gas is greater than unity at high pressure and temperature? - Quora