42g of N₂ react with excess of O₂ to produce NO. Amount of NO
4.5 (670) · $ 19.00 · In stock
Share your videos with friends, family, and the world
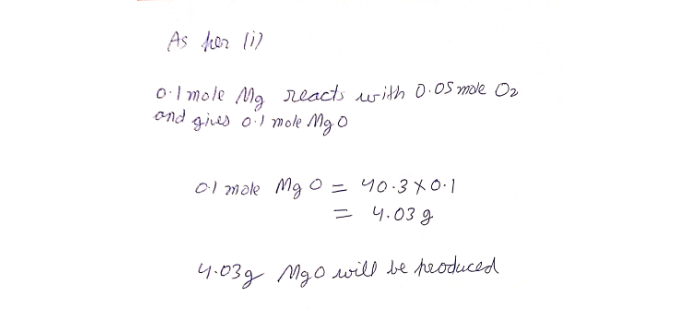
Answered: Suppose 2.43 g of magnesium is reacted…
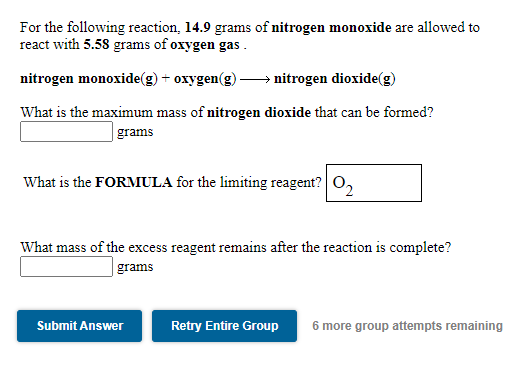
Solved For the following reaction. 14.9 grams of nitrogen
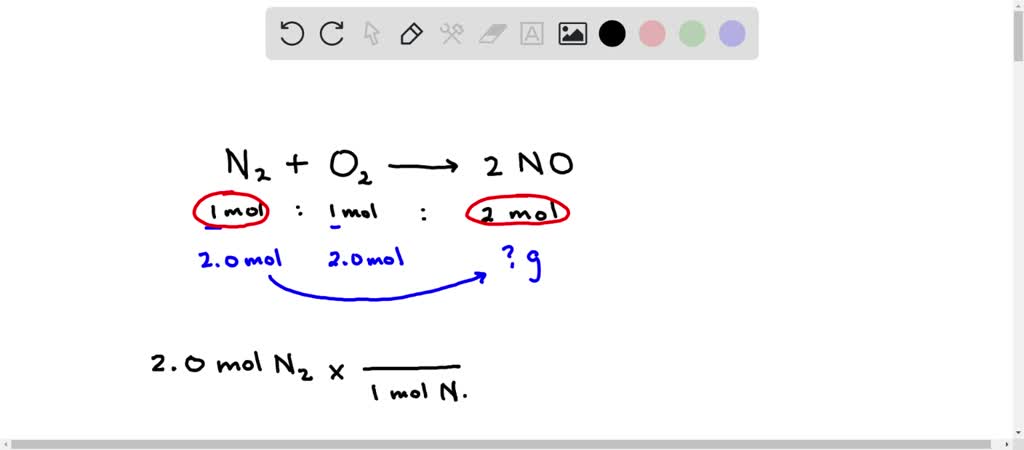
SOLVED: Given the balanced reaction: N2 + O2 → 2NO. How many grams of NO are produced from the reaction of 2.0 mol of N2 with 2.0 mol of O2? Select one

27 g Al reacts completely with how many grams Oxygen.

Mole Concept PDF, PDF, Mole (Unit)
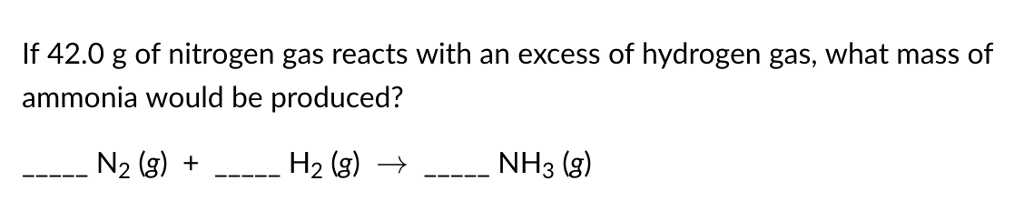
Solved If 42.0 g of nitrogen gas reacts with an excess of
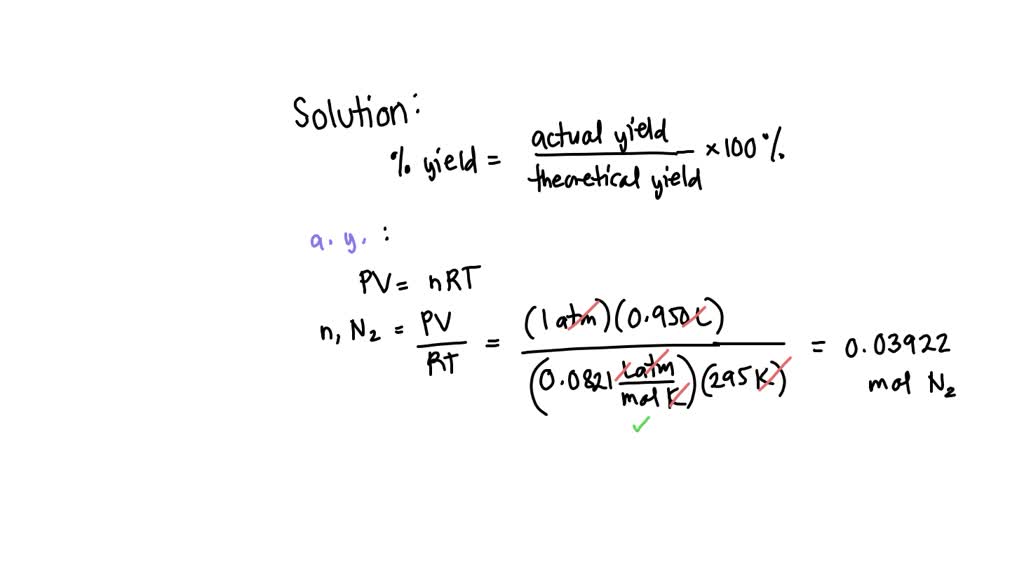
SOLVED: A mass of 6.45 g N2H4(g) reacts with excess oxygen. If 13.5 g NO2(g) is collected, what is the percent yield of the reaction? N2H4(g) + 3 O2(g) → 2 NO2(g) +
Solved If 42.0 g of nitrogen gas reacts with an excess of
31g of CuCO3 on heating give 16g of CuO. what is %age yield of reaction. 80% 85% 75% 50%

stoy-key-ahm-e-tree) - ppt download